TEVA PHARMACEUTICALS USA, INC: Teva Announces Updated Indication and Vial Presentation for GRANIX® (tbo-filgrastim) Injection in United States | American Pharmacy News

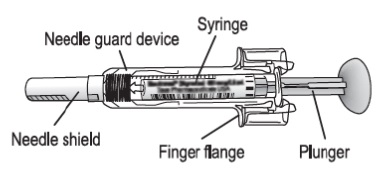
Granix® Hematopoietic Agent TBO-Filgrastim, Preservative Free 480 mcg / 0.8 mL Subcutaneous Injection Prefilled Syringe 5 Syringes
GRANIX (TBO-FILGRASTIM) INJECTION Trademark of TEVA Pharmaceutical Industries Limited Serial Number: 86040042 :: Trademarkia Trademarks

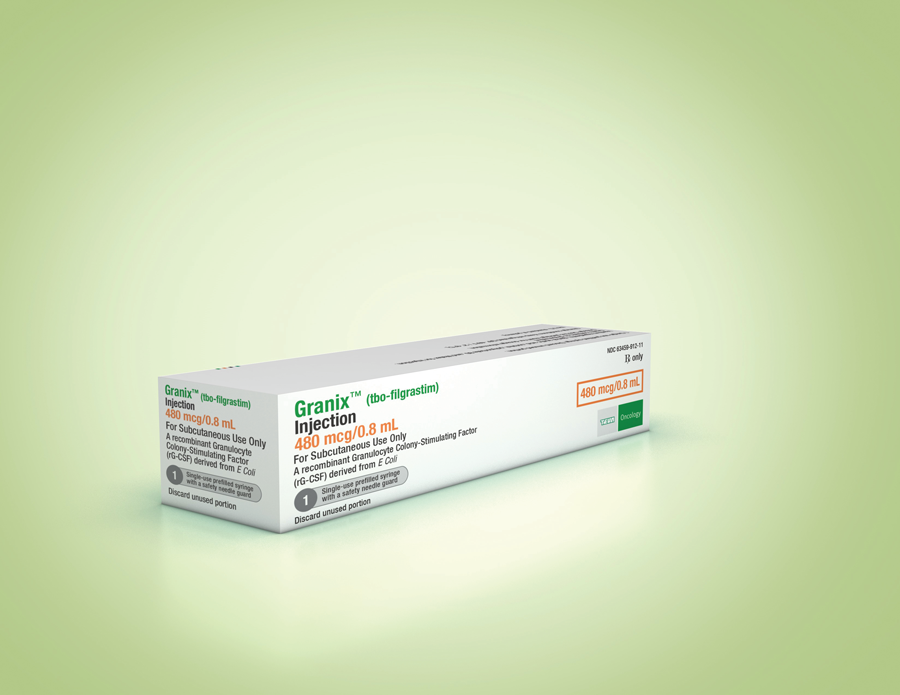
These highlights do not include all the information needed to use GRANIX safely and effectively. See full prescribing information for GRANIX.GRANIX® (tbo-filgrastim) injection, for subcutaneous use Initial U.S. Approval: 2012

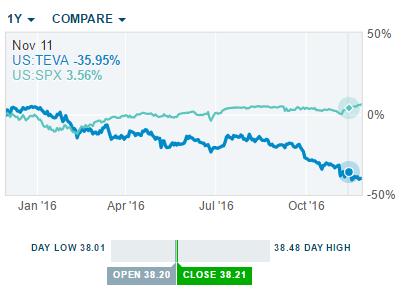
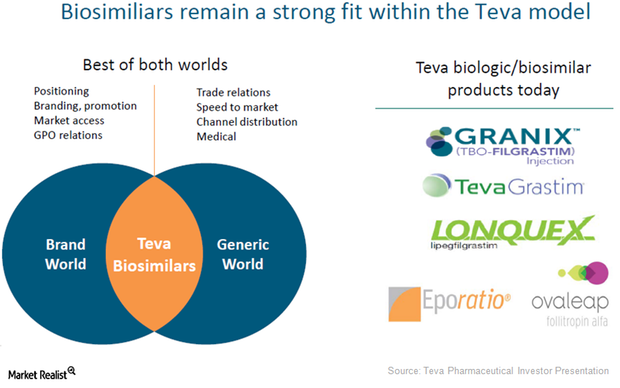
Market Share by Filgrastim Brand (As a Percentage of Sales Before Rebates) | Download Scientific Diagram